Markets
News
Analysis
User
24/7
Economic Calendar
Education
Data
- Names
- Latest
- Prev













Signal Accounts for Members
All Signal Accounts
All Contests


[Ethereum Drops Below $2100] February 5Th, According To Htx Market Data, Ethereum Fell Below $2,100, With A 24-Hour Percentage Decrease Expanding To 8.66%
[Minneapolis Mayor Calls For End To Federal Immigration Enforcement] On April 4, Local Time, In Response To US President Trump's Statement That Federal Immigration Enforcement Needed A "more Lenient Approach," Minneapolis Mayor Jacob Frey Said That Such A Change Was Welcome. However, He Emphasized That The Presence Of 2,000 Federal Law Enforcement Officers In Minneapolis Is Still Insufficient To Ease The Situation, And The Federal Government Should Terminate Its Immigration Enforcement Operations In The City
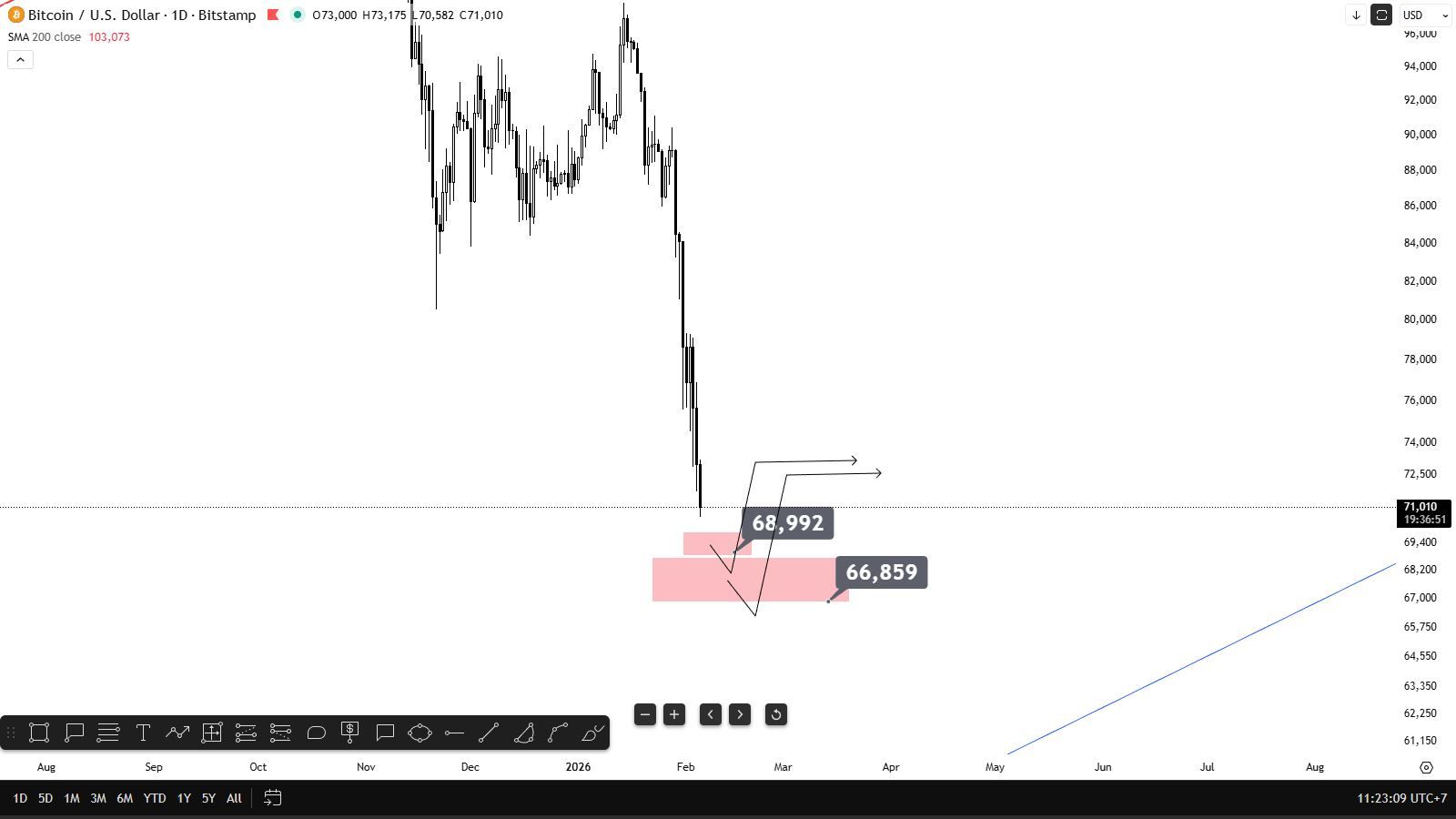
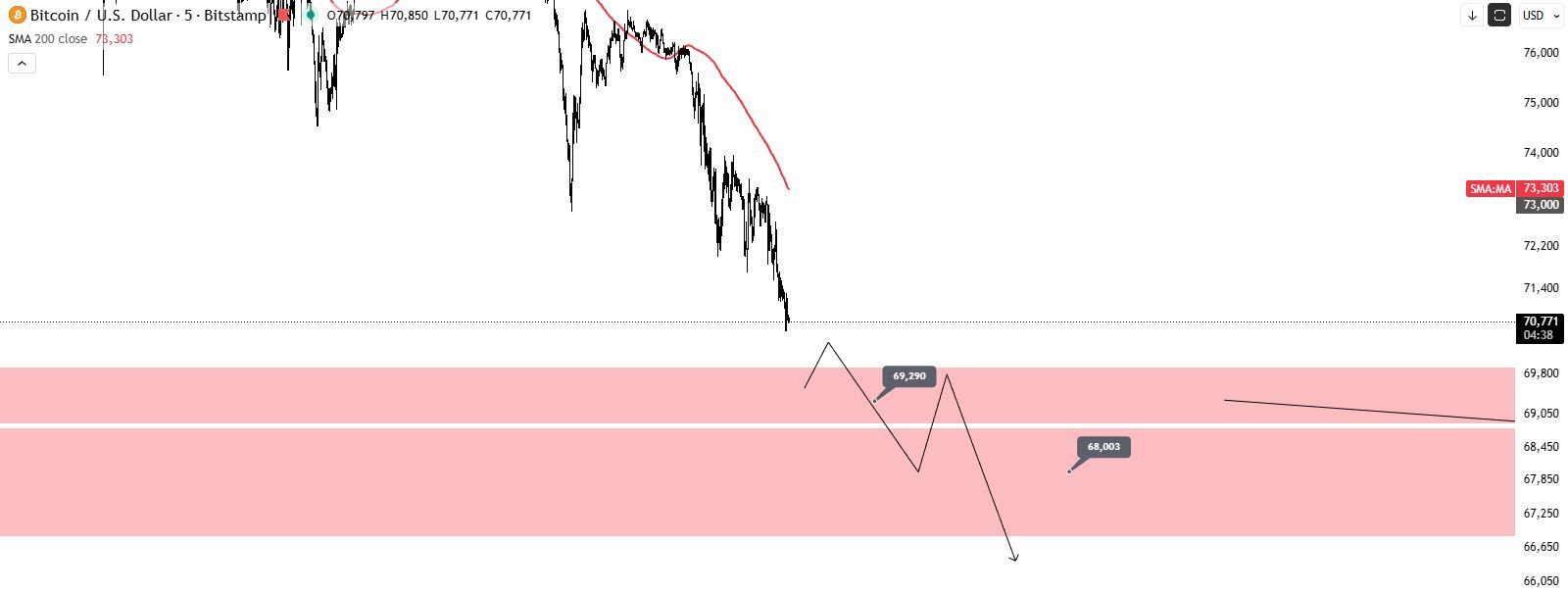
[Bitcoin Drops Below $71,000] February 5Th, According To Htx Market Data, Bitcoin Fell Below $71,000, With A 24-Hour Decline Expanding To 7.56%
Spot Silver Continued Its Decline, With Intraday Losses Widening To 15%, Currently Trading At $74.86 Per Ounce
The Thailand Futures Exchange (TFEX) Has Announced A Temporary Suspension Of Online Trading In Silver Futures

 Euro Zone Core CPI Prelim YoY (Jan)
Euro Zone Core CPI Prelim YoY (Jan)A:--
F: --
P: --
 Euro Zone Core HICP Prelim YoY (Jan)
Euro Zone Core HICP Prelim YoY (Jan)A:--
F: --
P: --
 Euro Zone HICP Prelim YoY (Jan)
Euro Zone HICP Prelim YoY (Jan)A:--
F: --
P: --
 Euro Zone PPI MoM (Dec)
Euro Zone PPI MoM (Dec)A:--
F: --
 Euro Zone Core HICP Prelim MoM (Jan)
Euro Zone Core HICP Prelim MoM (Jan)A:--
F: --
P: --
 Italy HICP Prelim YoY (Jan)
Italy HICP Prelim YoY (Jan)A:--
F: --
P: --
 Euro Zone Core CPI Prelim MoM (Jan)
Euro Zone Core CPI Prelim MoM (Jan)A:--
F: --
P: --
 Euro Zone PPI YoY (Dec)
Euro Zone PPI YoY (Dec)A:--
F: --
 U.S. MBA Mortgage Application Activity Index WoW
U.S. MBA Mortgage Application Activity Index WoWA:--
F: --
P: --
 Brazil IHS Markit Composite PMI (Jan)
Brazil IHS Markit Composite PMI (Jan)A:--
F: --
P: --
 Brazil IHS Markit Services PMI (Jan)
Brazil IHS Markit Services PMI (Jan)A:--
F: --
P: --
 U.S. ADP Employment (Jan)
U.S. ADP Employment (Jan)A:--
F: --
 The U.S. Treasury Department released its quarterly refinancing statement.
The U.S. Treasury Department released its quarterly refinancing statement. U.S. IHS Markit Composite PMI Final (Jan)
U.S. IHS Markit Composite PMI Final (Jan)A:--
F: --
P: --
 U.S. IHS Markit Services PMI Final (Jan)
U.S. IHS Markit Services PMI Final (Jan)A:--
F: --
P: --
 U.S. ISM Non-Manufacturing Price Index (Jan)
U.S. ISM Non-Manufacturing Price Index (Jan)A:--
F: --
P: --
 U.S. ISM Non-Manufacturing Employment Index (Jan)
U.S. ISM Non-Manufacturing Employment Index (Jan)A:--
F: --
P: --
 U.S. ISM Non-Manufacturing New Orders Index (Jan)
U.S. ISM Non-Manufacturing New Orders Index (Jan)A:--
F: --
P: --
 U.S. ISM Non-Manufacturing Inventories Index (Jan)
U.S. ISM Non-Manufacturing Inventories Index (Jan)A:--
F: --
P: --
 U.S. ISM Non-Manufacturing PMI (Jan)
U.S. ISM Non-Manufacturing PMI (Jan)A:--
F: --
P: --
 U.S. EIA Weekly Crude Oil Imports Changes
U.S. EIA Weekly Crude Oil Imports ChangesA:--
F: --
P: --
 U.S. EIA Weekly Heating Oil Stock Changes
U.S. EIA Weekly Heating Oil Stock ChangesA:--
F: --
P: --
 U.S. EIA Weekly Crude Demand Projected by Production
U.S. EIA Weekly Crude Demand Projected by ProductionA:--
F: --
P: --
 U.S. EIA Weekly Gasoline Stocks Change
U.S. EIA Weekly Gasoline Stocks ChangeA:--
F: --
P: --
 U.S. EIA Weekly Crude Stocks Change
U.S. EIA Weekly Crude Stocks ChangeA:--
F: --
P: --
 U.S. EIA Weekly Cushing, Oklahoma Crude Oil Stocks Change
U.S. EIA Weekly Cushing, Oklahoma Crude Oil Stocks ChangeA:--
F: --
P: --
 Australia Trade Balance (SA) (Dec)
Australia Trade Balance (SA) (Dec)A:--
F: --
 Australia Exports MoM (SA) (Dec)
Australia Exports MoM (SA) (Dec)A:--
F: --
 Japan 30-Year JGB Auction Yield
Japan 30-Year JGB Auction YieldA:--
F: --
P: --
 Indonesia Annual GDP Growth
Indonesia Annual GDP GrowthA:--
F: --
P: --
 Indonesia GDP YoY (Q4)
Indonesia GDP YoY (Q4)A:--
F: --
P: --
 France Industrial Output MoM (SA) (Dec)
France Industrial Output MoM (SA) (Dec)--
F: --
P: --
 Italy IHS Markit Construction PMI (Jan)
Italy IHS Markit Construction PMI (Jan)--
F: --
P: --
 Euro Zone IHS Markit Construction PMI (Jan)
Euro Zone IHS Markit Construction PMI (Jan)--
F: --
P: --
 Germany Construction PMI (SA) (Jan)
Germany Construction PMI (SA) (Jan)--
F: --
P: --
 Italy Retail Sales MoM (SA) (Dec)
Italy Retail Sales MoM (SA) (Dec)--
F: --
P: --
 U.K. Markit/CIPS Construction PMI (Jan)
U.K. Markit/CIPS Construction PMI (Jan)--
F: --
P: --
 France 10-Year OAT Auction Avg. Yield
France 10-Year OAT Auction Avg. Yield--
F: --
P: --
 Euro Zone Retail Sales YoY (Dec)
Euro Zone Retail Sales YoY (Dec)--
F: --
P: --
 Euro Zone Retail Sales MoM (Dec)
Euro Zone Retail Sales MoM (Dec)--
F: --
P: --
 U.K. BOE MPC Vote Cut (Feb)
U.K. BOE MPC Vote Cut (Feb)--
F: --
P: --
 U.K. BOE MPC Vote Hike (Feb)
U.K. BOE MPC Vote Hike (Feb)--
F: --
P: --
 U.K. BOE MPC Vote Unchanged (Feb)
U.K. BOE MPC Vote Unchanged (Feb)--
F: --
P: --
 U.K. Benchmark Interest Rate
U.K. Benchmark Interest Rate--
F: --
P: --
 MPC Rate Statement
MPC Rate Statement U.S. Challenger Job Cuts (Jan)
U.S. Challenger Job Cuts (Jan)--
F: --
P: --
 U.S. Challenger Job Cuts MoM (Jan)
U.S. Challenger Job Cuts MoM (Jan)--
F: --
P: --
 U.S. Challenger Job Cuts YoY (Jan)
U.S. Challenger Job Cuts YoY (Jan)--
F: --
P: --
 Bank of England Governor Bailey held a press conference on monetary policy.
Bank of England Governor Bailey held a press conference on monetary policy. Euro Zone ECB Marginal Lending Rate
Euro Zone ECB Marginal Lending Rate--
F: --
P: --
 Euro Zone ECB Deposit Rate
Euro Zone ECB Deposit Rate--
F: --
P: --
 Euro Zone ECB Main Refinancing Rate
Euro Zone ECB Main Refinancing Rate--
F: --
P: --
 ECB Monetary Policy Statement
ECB Monetary Policy Statement U.S. Weekly Initial Jobless Claims (SA)
U.S. Weekly Initial Jobless Claims (SA)--
F: --
P: --
 U.S. Initial Jobless Claims 4-Week Avg. (SA)
U.S. Initial Jobless Claims 4-Week Avg. (SA)--
F: --
P: --
 U.S. Weekly Continued Jobless Claims (SA)
U.S. Weekly Continued Jobless Claims (SA)--
F: --
P: --
 ECB Press Conference
ECB Press Conference U.S. JOLTS Job Openings (SA) (Dec)
U.S. JOLTS Job Openings (SA) (Dec)--
F: --
P: --
 U.S. EIA Weekly Natural Gas Stocks Change
U.S. EIA Weekly Natural Gas Stocks Change--
F: --
P: --
 BOC Gov Macklem Speaks
BOC Gov Macklem Speaks
















































No matching data
View All

No data





The end of the earnings season is always a good time to take a step back and see who shined (and who not so much). Let’s take a look at how therapeutics stocks fared in Q3, starting with United Therapeutics .
Over the next few years, therapeutic companies, which develop a wide variety of treatments for diseases and disorders, face strong tailwinds from advancements in precision medicine (including the use of AI to improve hit rates) and growing demand for treatments targeting rare diseases. However, headwinds such as rising scrutiny over drug pricing, regulatory unknowns, and competition from larger, more resourced pharmaceutical companies could weigh on growth.
The 11 therapeutics stocks we track reported a mixed Q3. As a group, revenues beat analysts’ consensus estimates by 10.5%.
Luckily, therapeutics stocks have performed well with share prices up 16.4% on average since the latest earnings results.
Founded by a mother seeking treatment for her daughter's pulmonary arterial hypertension, United Therapeutics develops and commercializes medications for chronic lung diseases and other life-threatening conditions, with a focus on pulmonary hypertension treatments.
United Therapeutics reported revenues of $799.5 million, up 6.8% year on year. This print fell short of analysts’ expectations by 1.6%. Overall, it was a softer quarter for the company with a miss of analysts’ revenue estimates and a significant miss of analysts’ EPS estimates.
"Our commercial and clinical teams continue to deliver record results, validating our strategic objectives," said Martine Rothblatt, Chairperson and Chief Executive Officer of United Therapeutics.
United Therapeutics delivered the weakest performance against analyst estimates of the whole group. Interestingly, the stock is up 12.3% since reporting and currently trades at $466.42.
Best Q3: Halozyme Therapeutics
Known for transforming hours-long intravenous infusions into minutes-long subcutaneous injections, Halozyme Therapeutics develops and licenses its proprietary ENHANZE technology that enables subcutaneous delivery of injectable drugs that would otherwise require intravenous administration.
Halozyme Therapeutics reported revenues of $354.3 million, up 22.1% year on year, outperforming analysts’ expectations by 3.1%. The business had an exceptional quarter with full-year EBITDA guidance exceeding analysts’ expectations and an impressive beat of analysts’ full-year EPS guidance estimates.
Halozyme Therapeutics delivered the fastest revenue growth among its peers. The market seems happy with the results as the stock is up 10.7% since reporting. It currently trades at $73.27.
Pioneering treatments for conditions that often had no previous therapeutic options, BioMarin Pharmaceutical develops and commercializes therapies that address the root causes of rare genetic disorders, particularly those affecting children.
BioMarin Pharmaceutical reported revenues of $776.1 million, up 4.1% year on year, in line with analysts’ expectations. It was a softer quarter as it posted a significant miss of analysts’ full-year EPS guidance estimates and a significant miss of analysts’ EPS estimates.
Interestingly, the stock is up 8.4% since the results and currently trades at $57.12.
Read our full analysis of BioMarin Pharmaceutical’s results here.
Founded in 1989 with a mission to create medicines that treat the underlying causes of disease rather than just symptoms, Vertex Pharmaceuticals develops and markets transformative medicines for serious diseases, with a focus on cystic fibrosis, sickle cell disease, and pain management.
Vertex Pharmaceuticals reported revenues of $3.08 billion, up 11% year on year. This print was in line with analysts’ expectations. Aside from that, it was a mixed quarter as it also produced a beat of analysts’ EPS estimates but full-year revenue guidance meeting analysts’ expectations.
The stock is up 11.5% since reporting and currently trades at $474.84.
Read our full, actionable report on Vertex Pharmaceuticals here, it’s free.
Founded in 1978 and pioneering treatments for some of medicine's most complex challenges, Biogen develops and markets therapies for neurological conditions, including multiple sclerosis, Alzheimer's disease, spinal muscular atrophy, and rare diseases.
Biogen reported revenues of $2.53 billion, up 2.8% year on year. This result topped analysts’ expectations by 8.6%. Overall, it was a strong quarter as it also logged an impressive beat of analysts’ revenue estimates and a beat of analysts’ EPS estimates.
The stock is up 19.1% since reporting and currently trades at $176.16.
Shares of Halozyme Therapeutics, Inc. (HALO) rallied 3% on Wednesday after the company announced preliminary 2025 estimates above Wall Street expectations. The company also announced the acquisition of Surf Bio.
The biopharmaceutical company said that it now expects 2025 total revenue of $1.385 - $1.4 billion, representing a year-on-year growth of 36% to 38%, and exceeding a Wall Street estimate of $1.34 billion.
Year Ahead
The company also raised its 2026 revenue guidance to $1.71 - $1.81 billion, representing growth of 23% to 30%, higher than an analyst estimate of $1.69 billion, backing on a sharp increase in royalty revenue. The company had previously predicted 2026 revenue in the range of $1.43 to $1.53 billion.
The company now expects adjusted earnings per share of $7.75 - $8.25 for 2026, up from its previous estimate of $6.50 to $7.00, and in line with an analyst estimate of $8.21.
"Our increased multi-year guidance reflects both the strength of our core ENHANZE (drug delivery technology) business and the exceptional momentum we built in 2025,” CEO Helen Torley said.
“By the end of 2026, we project we will have 15 partner programs in development and have signed three or more new drug delivery licensing agreements, expanding the reach and growing our opportunity through our diversified drug delivery portfolio,” Torley added.
Surf Bio Acquisition
Halozyme on Wednesday also said that it acquired pharmaceutical firm Surf Bio for up to $400 million in December to expand its drug delivery opportunity.
The deal included an upfront payment of $300 million and milestone payments of up to $100 million.
How Did Stocktwits Users React?
On Stocktwits, retail sentiment around HALO stock rose from ‘bearish’ to ‘bullish’ territory over the past 24 hours, while message volume increased from ‘normal’ to ‘high’ levels.
A Stocktwits user voiced confidence in the company’s new CEO Helen Torley attracting a broader range of investors.
https://www.stocktwits.com/Sean29md/message/643129433
HALO stock has risen 29% over the past 12 months.
By Colin Kellaher
Biogen has won U.S. Food and Drug Administration breakthrough-therapy designation for its litifilimab drug candidate for the treatment of the chronic autoimmune disease cutaneous lupus erythematosus, or CLE.
Biogen on Wednesday said litifilimab has the potential to be a first-in-class therapy targeting blood dendritic cell antigen 2 in CLE, a skin disease that currently has no targeted treatments.
The FDA's breakthrough-therapy designation aims to expedite the development and review of a drug for serious conditions when preliminary clinical evidence shows the drug might offer substantial improvement over available therapies.
Biogen said the litifilimab designation is based on data that includes Phase 2 study results showing improvements in CLE skin- disease activity.
The Cambridge, Mass., biotechnology company said it is continuing to evaluate the efficacy and safety of litifilimab in a Phase 3 study, with a data readout expected in 2027.
Write to Colin Kellaher at colin.kellaher@wsj.com
Revenue and royalty guidance for 2025 and 2026 have been raised, with 2026 royalty revenue now expected to surpass $1 billion a year earlier than previously projected. Strategic acquisitions, including Surf Bio, expand drug delivery capabilities and support long-term growth.
Original document: Halozyme Therapeutics, Inc. [HALO] Press release — Jan. 28 2026
Raised 2025 and 2026 revenue guidance, with royalty revenue growth projected to exceed 50% in 2025 and 30–35% in 2026. Strategic acquisitions of Surf Bio and Elektrofi expand the drug delivery portfolio and extend IP protection, supporting long-term growth.
Original document: Halozyme Therapeutics, Inc. [HALO] Press release — Jan. 28 2026
CAMBRIDGE, Mass., Jan. 28, 2026 (GLOBE NEWSWIRE) -- Biogen Inc. – announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation for litifilimab (BIIB059) for the treatment of cutaneous lupus erythematosus (CLE). Litifilimab is a first in-class, humanized IgG1 monoclonal antibody (mAb) targeting blood dendritic cell antigen 2 (BDCA2). CLE is a chronic autoimmune disease affecting the skin that currently has no targeted treatments.
"The breakthrough therapy designation for litifilimab illustrates the FDA’s recognition of cutaneous lupus as a serious disease that urgently requires new therapies," said Victoria Werth, MD, MS, a professor of Dermatology at the Perelman School of Medicine at the University of Pennsylvania, and one of the researchers who is conducting the phase 3 trial. "With topical steroids and antimalarials as the initial therapies for managing CLE and no alternatives specifically approved for CLE, there is a need for effective, targeted treatments, and that could be a drug like litifilimab.”
The designation is intended to expedite the development and review of drugs for serious conditions, and is based on the totality of litifilimab data, including the results from the Phase 2 LILAC study. The LILAC data were previously published in The New England Journal of Medicine and demonstrated that litifilimab reduced skin disease activity in people with CLE compared to placebo. The current standard of care for CLE includes topical steroids, antimalarials and immunosuppressants. While current treatments help manage symptoms, they do not alter the progression of the disease.
“The FDA grants breakthrough therapy designation to programs based on the seriousness of the condition and the potential of the therapeutic candidate to provide substantial improvements over available therapies. The FDA's designation reinforces Biogen’s belief that litifilimab could be a first-in-class therapy targeting BDCA2 for cutaneous lupus erythematosus,” said Priya Singhal, M.D., M.P.H., Executive Vice President and Head of Development at Biogen. “This designation is a significant milestone for litifilimab as we advance the ongoing AMETHYST Phase 3 study, with the goal of bringing a new potential therapeutic option to the millions of people living with CLE.”
Biogen is continuing to evaluate the efficacy and safety of litifilimab in the AMETHYST Phase 3 study, with a data readout expected in 2027. More information on the AMETHYST study (NCT05531565) is available at clinicaltrials.gov and BiogenTrialLink.
“The Lupus Research Alliance is dedicated to advancing lupus research, and today’s FDA Breakthrough Therapy designation for litifilimab reinforces our shared understanding of cutaneous lupus as a serious, debilitating condition that urgently needs therapies that can alter the course of the disease," said Albert T. Roy, President & CEO of the Lupus Research Alliance. "Incorporating the voices of people living with cutaneous lupus is vital to advancing drug development, and through our clinical affiliate, Lupus Therapeutics, we are proud to collaborate with Biogen on the cutaneous lupus erythematosus clinical trials for litifilimab. As a convenor bringing together leading industry partners, clinicians, patients, and FDA experts, the Lupus Research Alliance is encouraged by this progress to accelerate a potential new treatment that may improve the quality of life for those affected by CLE."
About Litifilimab (BIIB059)
Litifilimab (known as BIIB059), discovered and developed in-house by Biogen scientists, is a humanized IgG1 monoclonal antibody (mAb) targeting BDCA2 and is being investigated for the potential treatment of systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE). BDCA2 is a receptor that is predominantly expressed on a subset of human immune cells called Plasmacytoid Dendritic Cells (pDCs). Binding of litifilimab to BDCA2 has been shown to reduce production of pro-inflammatory molecules by pDCs, including type-I interferon (IFN-I) as well as other cytokines and chemokines.1,2 These pro-inflammatory mediators are thought to play a major role in the pathogenesis of systemic and cutaneous lupus.
Litifilimab is an investigational therapeutic candidate that has not yet been approved by any regulatory authority and its safety and effectiveness have not been established.
About Cutaneous Lupus Erythematosus (CLE)
CLE, a type of lupus, is a chronic autoimmune skin disease that can occur with or without systemic manifestations; people with CLE frequently experience symptoms including rash, pain, itch and photosensitivity as well as skin damage that may worsen over time and can include irreversible scarring, alopecia and dyspigmentation that can be disfiguring and substantially impact quality of life.3-6
Although anyone can develop lupus, an estimated 90 percent of people living with lupus are women; most begin to see symptoms between the ages of 15-40.7 The disease disproportionately impacts diverse ethno-racial groups, including African American, Asian, American Indian/Alaskan Native and Hispanic/Latino communities.8-10 There is currently no cure for lupus.
About Biogen
Founded in 1978, Biogen is a leading biotechnology company that pioneers innovative science to deliver new medicines to transform patients’ lives and to create value for shareholders and our communities. We apply deep understanding of human biology and leverage different modalities to advance first-in-class treatments or therapies that deliver superior outcomes. Our approach is to take bold risks, balanced with return on investment to deliver long-term growth.
We routinely post information that may be important to investors on our website at www.biogen.com. Follow us on social media - Facebook, LinkedIn, X, YouTube.
Biogen Safe Harbor
This news release contains forward-looking statements, including: the potential clinical effects of litifilimab; the potential of litifilimab to improve the health, wellbeing and outcomes for patients with CLE; the potential benefits, safety and efficacy of litifilimab; potential regulatory discussions, submissions and approvals and the timing thereof; potential therapeutic options for the treatment of CLE; the potential of Biogen's commercial business and pipeline programs, including litifilimab; and risks and uncertainties associated with drug development and commercialization. These forward-looking statements may be accompanied by such words as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “forecast,” “goal,” “guidance,” “hope,” “intend,” “may,” “objective,” “outlook,” “plan,” “possible,” “potential,” “predict,” “project,” “prospect,” “should,” “target,” “will,” “would” or the negative of these words or other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early-stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements. Given their forward-looking nature, these statements involve substantial risks and uncertainties that may be based on inaccurate assumptions and could cause actual results to differ materially from those reflected in such statements.
These forward-looking statements are based on management’s current beliefs and assumptions and on information currently available to management. Given their nature, we cannot assure that any outcome expressed in these forward-looking statements will be realized in whole or in part. We caution that these statements are subject to risks and uncertainties, many of which are outside of our control and could cause future events or results to differ materially from those stated or implied in this document, including, among others, uncertainty of our long-term success in developing, licensing, or acquiring other product candidates or additional indications for existing products; expectations, plans, prospects and timing of actions relating to product approvals, approvals of additional indications for our existing products, sales, pricing, growth, reimbursement and launch of our marketed and pipeline products; the potential impact of increased product competition in the biopharmaceutical and healthcare industry, as well as any other markets in which we compete, including increased competition from new originator therapies, generics, prodrugs and biosimilars of existing products and products approved under abbreviated regulatory pathways; our ability to effectively implement our corporate strategy; difficulties in obtaining and maintaining adequate coverage, pricing, and reimbursement for our products; the drivers for growing our business, including our dependence on collaborators and other third parties for the development, regulatory approval, and commercialization of products and other aspects of our business, which are outside of our full control; risks related to commercialization of biosimilars, which is subject to such risks related to our reliance on third-parties, intellectual property, competitive and market challenges and regulatory compliance; the risk that positive results in a clinical trial may not be replicated in subsequent or confirmatory trials or success in early stage clinical trials may not be predictive of results in later stage or large scale clinical trials or trials in other potential indications; risks associated with clinical trials, including our ability to adequately manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during clinical trials, regulatory authorities may require additional information or further studies, or may fail to approve or may delay approval of our drug candidates; and the occurrence of adverse safety events, restrictions on use with our products, or product liability claims; and any other risks and uncertainties that are described in other reports we have filed with the U.S. Securities and Exchange Commission, which are available on the SEC’s website at www.sec.gov.
These statements speak only as of the date of this press release and are based on information and estimates available to us at this time. Should known or unknown risks or uncertainties materialize or should underlying assumptions prove inaccurate, actual results could vary materially from past results and those anticipated, estimated or projected. Investors are cautioned not to put undue reliance on forward-looking statements. A further list and description of risks, uncertainties and other matters can be found in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024 and in our subsequent reports on Form 10-Q. Except as required by law, we do not undertake any obligation to publicly update any forward-looking statements whether as a result of any new information, future events, changed circumstances or otherwise.
Digital Media Disclosure
From time to time, we have used, or expect in the future to use, our investor relations website (investors.biogen.com), the Biogen LinkedIn account (linkedin.com/company/biogen-) and the Biogen X account (https://x.com/biogen) as a means of disclosing information to the public in a broad, non-exclusionary manner, including for purposes of the SEC’s Regulation Fair Disclosure (Reg FD). Accordingly, investors should monitor our investor relations website and these social media channels in addition to our press releases, SEC filings, public conference calls and websites, as the information posted on them could be material to investors.
References:





As the craze of earnings season draws to a close, here’s a look back at some of the most exciting (and some less so) results from Q3. Today, we are looking at therapeutics stocks, starting with United Therapeutics .
Over the next few years, therapeutic companies, which develop a wide variety of treatments for diseases and disorders, face strong tailwinds from advancements in precision medicine (including the use of AI to improve hit rates) and growing demand for treatments targeting rare diseases. However, headwinds such as rising scrutiny over drug pricing, regulatory unknowns, and competition from larger, more resourced pharmaceutical companies could weigh on growth.
The 11 therapeutics stocks we track reported a mixed Q3. As a group, revenues beat analysts’ consensus estimates by 10.5%.
Luckily, therapeutics stocks have performed well with share prices up 17.1% on average since the latest earnings results.
Founded by a mother seeking treatment for her daughter's pulmonary arterial hypertension, United Therapeutics develops and commercializes medications for chronic lung diseases and other life-threatening conditions, with a focus on pulmonary hypertension treatments.
United Therapeutics reported revenues of $799.5 million, up 6.8% year on year. This print fell short of analysts’ expectations by 1.6%. Overall, it was a softer quarter for the company with a miss of analysts’ revenue and EPS estimates.
"Our commercial and clinical teams continue to deliver record results, validating our strategic objectives," said Martine Rothblatt, Chairperson and Chief Executive Officer of United Therapeutics.
United Therapeutics delivered the weakest performance against analyst estimates of the whole group. Interestingly, the stock is up 15% since reporting and currently trades at $477.64.
Best Q3: Halozyme Therapeutics
Known for transforming hours-long intravenous infusions into minutes-long subcutaneous injections, Halozyme Therapeutics develops and licenses its proprietary ENHANZE technology that enables subcutaneous delivery of injectable drugs that would otherwise require intravenous administration.
Halozyme Therapeutics reported revenues of $354.3 million, up 22.1% year on year, outperforming analysts’ expectations by 3.1%. The business had an exceptional quarter with full-year EBITDA guidance exceeding analysts’ expectations and an impressive beat of analysts’ full-year EPS guidance estimates.
Halozyme Therapeutics achieved the fastest revenue growth among its peers. The market seems happy with the results as the stock is up 8% since reporting. It currently trades at $71.51.
Pioneering treatments for conditions that often had no previous therapeutic options, BioMarin Pharmaceutical develops and commercializes therapies that address the root causes of rare genetic disorders, particularly those affecting children.
BioMarin Pharmaceutical reported revenues of $776.1 million, up 4.1% year on year, in line with analysts’ expectations. It was a softer quarter as it posted a significant miss of analysts’ full-year EPS guidance estimates and a significant miss of analysts’ EPS estimates.
Interestingly, the stock is up 9.3% since the results and currently trades at $57.57.
Read our full analysis of BioMarin Pharmaceutical’s results here.
Pioneering a nanoparticle technology that mimics the molecular structure of disease pathogens, Novavax develops and commercializes protein-based vaccines for infectious diseases, with a primary focus on its COVID-19 vaccine and combination respiratory vaccine candidates.
Novavax reported revenues of $70.45 million, down 16.6% year on year. This result beat analysts’ expectations by 61%. Taking a step back, it was a mixed quarter as it also logged an impressive beat of analysts’ revenue estimates but a significant miss of analysts’ EPS estimates.
Novavax achieved the biggest analyst estimates beat among its peers. The stock is up 28% since reporting and currently trades at $9.85.
Read our full, actionable report on Novavax here, it’s free.
Founded in 1989 with a mission to create medicines that treat the underlying causes of disease rather than just symptoms, Vertex Pharmaceuticals develops and markets transformative medicines for serious diseases, with a focus on cystic fibrosis, sickle cell disease, and pain management.
Vertex Pharmaceuticals reported revenues of $3.08 billion, up 11% year on year. This print was in line with analysts’ expectations. Aside from that, it was a mixed quarter as it also recorded a beat of analysts’ EPS estimates but full-year revenue guidance meeting analysts’ expectations.
The stock is up 11.7% since reporting and currently trades at $475.70.
Read our full, actionable report on Vertex Pharmaceuticals here, it’s free.
The risk of loss in trading financial instruments such as stocks, FX, commodities, futures, bonds, ETFs and crypto can be substantial. You may sustain a total loss of the funds that you deposit with your broker. Therefore, you should carefully consider whether such trading is suitable for you in light of your circumstances and financial resources.
No decision to invest should be made without thoroughly conducting due diligence by yourself or consulting with your financial advisors. Our web content might not suit you since we don't know your financial conditions and investment needs. Our financial information might have latency or contain inaccuracy, so you should be fully responsible for any of your trading and investment decisions. The company will not be responsible for your capital loss.
Without getting permission from the website, you are not allowed to copy the website's graphics, texts, or trademarks. Intellectual property rights in the content or data incorporated into this website belong to its providers and exchange merchants.
Not Logged In
Log in to access more features


Log In
Sign Up